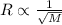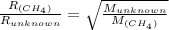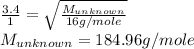Chemistry, 03.11.2020 21:00 cpcoolestkid4
Explain the relationship between the rate of effusion of a gas and its molar mass. Methane gas (CH4) effuses 3.4 times faster than an unknown gas. Determine the molar mass of the unknown gas. Show your work or explain your answer, giving specific values used to determine the answer.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 1

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

You know the right answer?
Explain the relationship between the rate of effusion of a gas and its molar mass.
Methane gas (CH4...
Questions


Social Studies, 15.04.2020 16:30





Mathematics, 15.04.2020 16:30

Mathematics, 15.04.2020 16:30











Mathematics, 15.04.2020 16:31

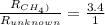
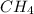 = 16 g/mole
= 16 g/mole