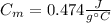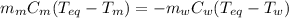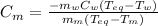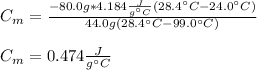Chemistry, 03.11.2020 17:20 Justadumbemo
A 44.0 g sample of an unknown metal at 99.0 oC was placed in a constant-pressure calorimeter of negligible heat capacity containing 80.0 mL water at 24.0 oC. The final temperature of the system was found to be 28.4 oC. Calculate the Specific heat of the metal if density of water is 1.00 g/ml.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
What pressure will be exerted by 0.675 moles of a gas at 25*c if it is in a 0.750-l container?
Answers: 1

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

You know the right answer?
A 44.0 g sample of an unknown metal at 99.0 oC was placed in a constant-pressure calorimeter of negl...
Questions

Geography, 17.05.2021 20:30

World Languages, 17.05.2021 20:30

Geography, 17.05.2021 20:30





English, 17.05.2021 20:40


English, 17.05.2021 20:40

Advanced Placement (AP), 17.05.2021 20:40





Health, 17.05.2021 20:40



Mathematics, 17.05.2021 20:40