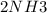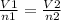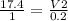Chemistry, 03.11.2020 17:00 diamoniquepowel
A sample of gas contains 0.1000 mol of N2(g) and 0.3000 mol of H2(g) and occupies a volume of 17.4 L. The following reaction takes place: N2(g) + 3H2(g)2NH3(g) Calculate the volume of the sample after the reaction takes place, assuming that the temperature and the pressure remain constant.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2
You know the right answer?
A sample of gas contains 0.1000 mol of N2(g) and 0.3000 mol of H2(g) and occupies a volume of 17.4 L...
Questions


Chemistry, 12.01.2021 22:40



Mathematics, 12.01.2021 22:40

Mathematics, 12.01.2021 22:40


Mathematics, 12.01.2021 22:40


Mathematics, 12.01.2021 22:40

Mathematics, 12.01.2021 22:40


Mathematics, 12.01.2021 22:40


Mathematics, 12.01.2021 22:40


Mathematics, 12.01.2021 22:40

Mathematics, 12.01.2021 22:40

Mathematics, 12.01.2021 22:40

 →
→