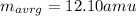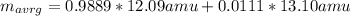Chemistry, 03.11.2020 17:00 mooredollie
An element has two common isotopes. 98.89% of its atoms have an atomic mass of 12.09 amu, whereas the other 1.11% have an atomic mass of 13.10 amu. Using the isotopic composition provided, calculate the average atomic mass of the element.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 04:00
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

You know the right answer?
An element has two common isotopes. 98.89% of its atoms have an atomic mass of 12.09 amu, whereas th...
Questions



Computers and Technology, 27.04.2021 20:10


Physics, 27.04.2021 20:10


Mathematics, 27.04.2021 20:10



Mathematics, 27.04.2021 20:10

Chemistry, 27.04.2021 20:10

Chemistry, 27.04.2021 20:10


Physics, 27.04.2021 20:10

Mathematics, 27.04.2021 20:10

Mathematics, 27.04.2021 20:10

Mathematics, 27.04.2021 20:10