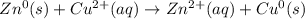Chemistry, 03.11.2020 16:50 bullockarwen
Identify the reduced substance, the oxidized substance, the reducing agent, and the oxidizing agent in the reaction of zinc with copper ions. Zn(s)+Cu2+(aq)⟶Zn2+(aq)+Cu(s) Which substance in the reaction gets reduced?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Temperature and kinetic energy are proportional. a) adirectly b) directly c) indirectly
Answers: 2

Chemistry, 22.06.2019 03:00
Which best describes how johannes kepler developed his laws of planetary motion
Answers: 3


Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2
You know the right answer?
Identify the reduced substance, the oxidized substance, the reducing agent, and the oxidizing agent...
Questions

Mathematics, 11.10.2019 19:00


History, 11.10.2019 19:00

Biology, 11.10.2019 19:00


Mathematics, 11.10.2019 19:00







Social Studies, 11.10.2019 19:00

Business, 11.10.2019 19:00

History, 11.10.2019 19:00

Mathematics, 11.10.2019 19:00


Health, 11.10.2019 19:00

Mathematics, 11.10.2019 19:00