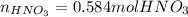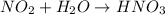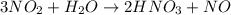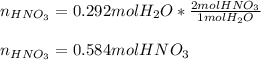Chemistry, 03.11.2020 16:20 zander434556
When nitrogen dioxide (NO2) from car exhaust combines with water in the air, it forms nitric acid (HNO3), which causes acid rain, and nitrogen oxide. Write the balanced chemical equation. How many moles of HNO3 are produced from 0.292 mole of H2O

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:10
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2


Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2

Chemistry, 23.06.2019 02:50
What is the typical rotational frequency frot for a molecule like n2 at room temperature (25∘c)? assume that d for this molecule is 1å=10−10m. take the total mass of an n2 molecule to be mn2=4.65×10−26kg. you will need to account for rotations around two axes (not just one) to find the correct frequency. express frot numerically in hertz, to three significant figures.
Answers: 3
You know the right answer?
When nitrogen dioxide (NO2) from car exhaust combines with water in the air, it forms nitric acid (H...
Questions





Mathematics, 16.11.2019 14:31

Mathematics, 16.11.2019 14:31

Chemistry, 16.11.2019 14:31

Mathematics, 16.11.2019 14:31

Chemistry, 16.11.2019 14:31

Physics, 16.11.2019 14:31

History, 16.11.2019 14:31








History, 16.11.2019 14:31

Physics, 16.11.2019 14:31