
Chemistry, 03.11.2020 14:00 ashleybarrera2000
Be sure to answer all parts.
Calculate the percent composition by mass (to 4 significant figures) of all the elements in calcium
phosphate (Ca3(PO4)2), a major component of bone.
% Ca
%P
% 0

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1



Chemistry, 23.06.2019 07:30
In a laboratory determination of the atomic weight of tin, a sample of tin is weighed in a crucible. nitric acid is added, and the reaction proceeds to give a hydrated tin(iv)oxide plus no2and h2o. the hydrated tin(iv)oxide is then heated strongly and reacts as follows: sno2.xh2o(s)sno2(s)+ xh2o(g)the sno2is finally cooled and weighed in the crucible. explain the effect on the calculated atomic weight of tin that would result from each of the following experimental errors: (a)considerable spattering occurs when the nitric acid is added to the tin.(b)the hydrated tin(iv)oxide is not heated sufficiently to change it completely to tin oxide.
Answers: 2
You know the right answer?
Be sure to answer all parts.
Calculate the percent composition by mass (to 4 significant figures) o...
Questions


Mathematics, 03.10.2019 05:20

Mathematics, 03.10.2019 05:20

History, 03.10.2019 05:20




Mathematics, 03.10.2019 05:20

English, 03.10.2019 05:20


Chemistry, 03.10.2019 05:20


Chemistry, 03.10.2019 05:20

English, 03.10.2019 05:20


Mathematics, 03.10.2019 05:20


Chemistry, 03.10.2019 05:20


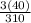 x 100 = 38.7%
x 100 = 38.7% 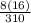 x 100 = 41.3%
x 100 = 41.3% 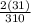 x 200 = 20%
x 200 = 20%


