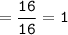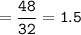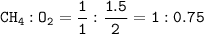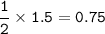Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1


Chemistry, 23.06.2019 01:10
A5.00 g of a in . g of at aa 5.00 g of b in . g of .?at .
Answers: 1

Chemistry, 23.06.2019 07:20
F1.5 mol of nabh4 react, how many moles of b2h6 are formed? 2 nabh4(aq) + h2so4(aq) → 2 h2(g) + na2so4(aq) + b2h6(g)
Answers: 1
You know the right answer?
How many grams of carbon dioxide are produced when 16.0
g of methane and 48.0 g of oxygen gas combu...
Questions

Mathematics, 06.03.2020 19:10

Chemistry, 06.03.2020 19:10


Computers and Technology, 06.03.2020 19:10






Biology, 06.03.2020 19:10

Computers and Technology, 06.03.2020 19:10



Biology, 06.03.2020 19:10


Computers and Technology, 06.03.2020 19:10