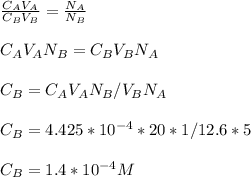Chemistry, 03.11.2020 03:20 sihamabdalla591
3.47 g of the hydrated "double salt", ammonium iron (II) sulfate hexahydrate, FeSO4(NH4)2SO4*6H2O was dissolved in 200. mL of water. 20.0 mL of the solution had some acid added to it and then it reacted completely with 12.6 mL of KMnO4 solution. Calculate the concentration of the KMnO4 solution given the full REDOX equation below. 5Fe2+ + MnO4- + 8H+ --> 5Fe3+ +Mn2+ + 4H2O

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1

Chemistry, 22.06.2019 23:00
What is the name of the enzyme that forms at the start of transcription?
Answers: 1

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1
You know the right answer?
3.47 g of the hydrated "double salt", ammonium iron (II) sulfate hexahydrate, FeSO4(NH4)2SO4*6H2O wa...
Questions


Biology, 08.03.2021 22:10

Mathematics, 08.03.2021 22:10

Mathematics, 08.03.2021 22:10

Chemistry, 08.03.2021 22:10

Health, 08.03.2021 22:10

Mathematics, 08.03.2021 22:10

Mathematics, 08.03.2021 22:10

Computers and Technology, 08.03.2021 22:10


Biology, 08.03.2021 22:10




Mathematics, 08.03.2021 22:10





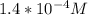
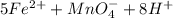 →
→ 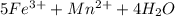
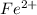 in FeSO₄(NH₄)₂SO₄*6H₂O
in FeSO₄(NH₄)₂SO₄*6H₂O 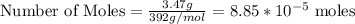
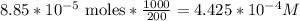
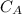 be concentration of
be concentration of 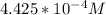
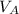 )= 20.0 ml
)= 20.0 ml
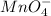 be
be 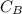 (the unknown)
(the unknown)
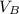 ) = 12.6 ml
) = 12.6 ml
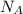 = 5 moles
= 5 moles
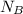 = 1 mole
= 1 mole