
Chemistry, 02.11.2020 21:00 SoccerdudeDylan
An aqueous magnesium chloride solution is made by dissolving 7.15 moles of MgCl2 in sufficient water so that the final volume of the solution is 2.50 L . Calculate the molarity of the MgCl2 solution.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Which is true of the reactants in this displacement reaction? fe + 2hcl fecl2 + h2 a. the reactants are located to the left of the arrow in the chemical equation. b. the reactants contain 1 iron atom, 2 hydrogen atoms, and 1 chlorine atom. c. the reactants are the atoms, molecules, or compounds formed in the reaction. d. the reactants have the same physical and chemical properties as the products.
Answers: 1

Chemistry, 21.06.2019 20:10
Why is the vapor pressure of a warm lake higher than the vapor pressure of a cold lake? o a. warm water has a greater heat of vaporization. ob. warm water evaporates more quickly. cool water evaporates more quickly. od. cool water has a greater heat of vaporization.
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3

You know the right answer?
An aqueous magnesium chloride solution is made by dissolving 7.15 moles of MgCl2 in sufficient water...
Questions


Mathematics, 20.09.2020 05:01

History, 20.09.2020 05:01


Chemistry, 20.09.2020 05:01

History, 20.09.2020 05:01

History, 20.09.2020 05:01

Physics, 20.09.2020 05:01

Mathematics, 20.09.2020 05:01


Biology, 20.09.2020 05:01


Mathematics, 20.09.2020 05:01

Mathematics, 20.09.2020 05:01

Mathematics, 20.09.2020 05:01


Mathematics, 20.09.2020 05:01

Mathematics, 20.09.2020 05:01


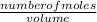
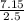 = 2.86mol/L
= 2.86mol/L

