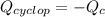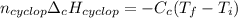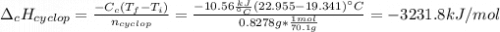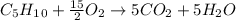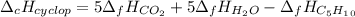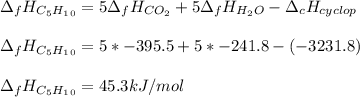Chemistry, 02.11.2020 16:50 uberagentkenny
Cyclopentene is a cyclic hydrocarbon like the ones used in the experiment. In another bomb calorimetry experiment 0.8278 g of cyclopentene is burned and the temperature of the calorimeter increased from 19.341C to 22.955C. The heat capacity of the calorimeter is 10.56 kJ C−1. Calculate the enthalpy of formation of cyclopentene in kJ/mol cyclopentene. Compare this to the accepted value of +32.6 kJ mol−1.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1



Chemistry, 22.06.2019 14:30
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3
You know the right answer?
Cyclopentene is a cyclic hydrocarbon like the ones used in the experiment. In another bomb calorimet...
Questions

History, 02.09.2019 08:00

Physics, 02.09.2019 08:00

English, 02.09.2019 08:00


Biology, 02.09.2019 08:00

Social Studies, 02.09.2019 08:00


Geography, 02.09.2019 08:00


English, 02.09.2019 08:00

Spanish, 02.09.2019 08:00


History, 02.09.2019 08:00

Social Studies, 02.09.2019 08:00

Chemistry, 02.09.2019 08:00

Mathematics, 02.09.2019 08:00


Mathematics, 02.09.2019 08:00

Biology, 02.09.2019 08:00

English, 02.09.2019 08:00