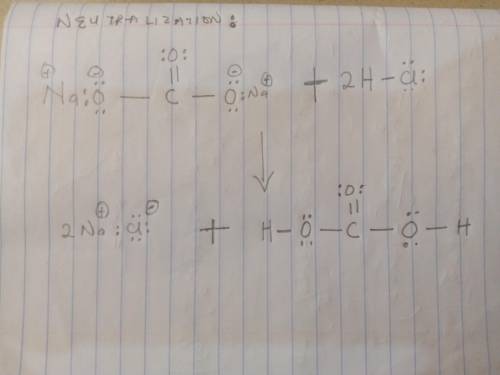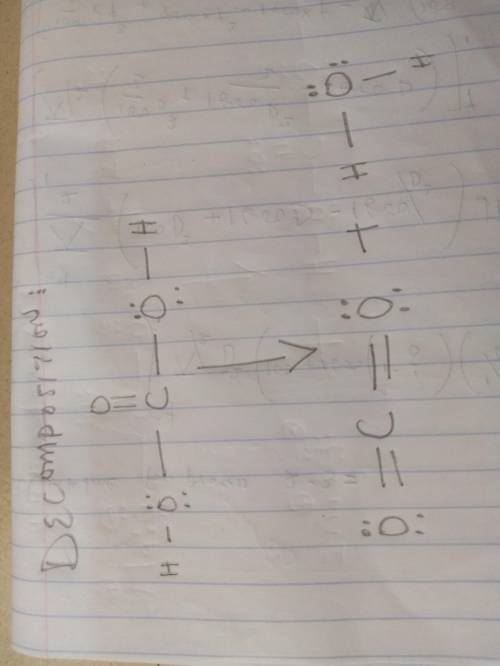Sodium bicarbonate is used to neutralize the remaining HCl at the end of the reaction. The initial products of this reaction are carbonic acid and sodium chloride. Carbonic acid then decomposes into carbon dioxide and water. Write a balanced chemical equation for each of these processes using Lewis structures (no molecular formulas).

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 01:10
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3
You know the right answer?
Sodium bicarbonate is used to neutralize the remaining HCl at the end of the reaction. The initial p...
Questions

Mathematics, 30.11.2020 20:50


History, 30.11.2020 20:50

Mathematics, 30.11.2020 20:50

Mathematics, 30.11.2020 20:50

Mathematics, 30.11.2020 20:50

Business, 30.11.2020 20:50

Mathematics, 30.11.2020 20:50



Biology, 30.11.2020 20:50








Mathematics, 30.11.2020 20:50