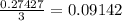Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 00:20
What type of context clue you understand the meaning of quandary?
Answers: 3

Chemistry, 23.06.2019 05:30
Suppose you discovered a new element with 120 protons and 2 electrons in its outer level . i'm what group does this new element belong? what properties would you expect it to have
Answers: 1

Chemistry, 23.06.2019 13:00
Sort these isotopes by whether they are most likely to undergo fusion or fission. hydrogen-3, uranium-233, plutonium-239, hydrogen-1, helium-3, plutonium-241
Answers: 2

Chemistry, 23.06.2019 14:00
What can happen to an atoms electrons when an electric current is passed through the atom?
Answers: 1
You know the right answer?
How many moles of BCl3 are needed to produce 10.0 g of HCl(aq) in the following reaction? (HCl molar...
Questions

Biology, 26.09.2019 02:30

English, 26.09.2019 02:30

Social Studies, 26.09.2019 02:30


Social Studies, 26.09.2019 02:30

Mathematics, 26.09.2019 02:30





Mathematics, 26.09.2019 02:30

English, 26.09.2019 02:30



Mathematics, 26.09.2019 02:30



Mathematics, 26.09.2019 02:30

English, 26.09.2019 02:30

Mathematics, 26.09.2019 02:30

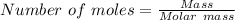
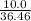
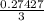 mole of BCl₃ would be needed to produce 0.27427 mole of HCl
mole of BCl₃ would be needed to produce 0.27427 mole of HCl