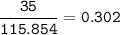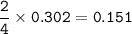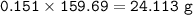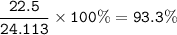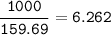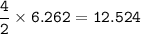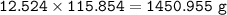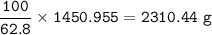Chemistry, 02.11.2020 06:30 Svetakotok
4 FeCO3 + O2 --> 2 Fe2O3 + 4CO2
a) A 35.0 g sample of pure FeCO3 produces 22.5 g of Fe2O3. What is the percentage yield of the reaction?
b) What mass of FeCO3 with a purity of 62.8% is needed to make 1.00 kg of Fe2O3?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 23.06.2019 01:00
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1

You know the right answer?
4 FeCO3 + O2 --> 2 Fe2O3 + 4CO2
a) A 35.0 g sample of pure FeCO3 produces 22.5 g of Fe2O3. What...
Questions



Mathematics, 28.09.2019 17:10


English, 28.09.2019 17:10




Social Studies, 28.09.2019 17:10








Chemistry, 28.09.2019 17:10

Mathematics, 28.09.2019 17:10

English, 28.09.2019 17:10

Mathematics, 28.09.2019 17:10