
Chemistry, 02.11.2020 05:50 ghari112345
PLEASE HELP! According to the equation, how many liters of oxygen gas at STP are produced when 2.00 moles of potassium chlorate decomposes?
a. 134 L
b. 67.2 L
c. 22.4 L
d. 11.2 L
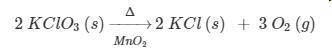

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Maria wants to determine which type of disinfectant kills the most bacteria. which of the following is the best way for maria to determine this? a. ask ten different companies that make disinfectants which type is best. b. put the same amount and species of bacteria on ten identical plates, and add ten different kinds of disinfectant to each plate. c. interview ten different people to determine which type of disinfectant they prefer. d. put the same amount and species of bacteria on ten identical plates, and add a different disinfectant to each plate.
Answers: 1

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1

Chemistry, 23.06.2019 02:00
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1
You know the right answer?
PLEASE HELP! According to the equation, how many liters of oxygen gas at STP are produced when 2.00...
Questions

History, 25.06.2019 11:40


History, 25.06.2019 11:40

Mathematics, 25.06.2019 11:40


Mathematics, 25.06.2019 11:40





Business, 25.06.2019 11:40


Biology, 25.06.2019 11:40



Biology, 25.06.2019 11:40


Mathematics, 25.06.2019 11:40

Mathematics, 25.06.2019 11:40



