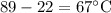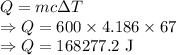Chemistry, 02.11.2020 05:00 rafamoreura
A red hot iron bar is dipped into 600 ml of water ( 600.g of water) which causes
the temperature of the water to rise from 22 degrees celcius to 89 degrees
celcius. Determine the heat lost by the iron bar. hint: The amount of heat lost
= amount of heat gained

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 12:40
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

You know the right answer?
A red hot iron bar is dipped into 600 ml of water ( 600.g of water) which causes
the temperature of...
Questions

Mathematics, 25.01.2022 17:40

English, 25.01.2022 17:40

Mathematics, 25.01.2022 17:40



Arts, 25.01.2022 17:40

Mathematics, 25.01.2022 17:40

Mathematics, 25.01.2022 17:40

Arts, 25.01.2022 17:40

Mathematics, 25.01.2022 17:40


Social Studies, 25.01.2022 17:50




Health, 25.01.2022 17:50

English, 25.01.2022 17:50

Chemistry, 25.01.2022 17:50


History, 25.01.2022 17:50

 = Change in temperature of water =
= Change in temperature of water =