

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1
You know the right answer?
Calculate the energy of the violet light emitted by a hydrogen atom with a wavelength of 410.1 nm....
Questions



Mathematics, 29.08.2019 01:20

English, 29.08.2019 01:20

Mathematics, 29.08.2019 01:20

Mathematics, 29.08.2019 01:20

Mathematics, 29.08.2019 01:20








Mathematics, 29.08.2019 01:20


History, 29.08.2019 01:20

Mathematics, 29.08.2019 01:20

Advanced Placement (AP), 29.08.2019 01:20

Mathematics, 29.08.2019 01:20

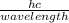
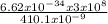 = 4.85 x 10⁻¹⁹J
= 4.85 x 10⁻¹⁹J

