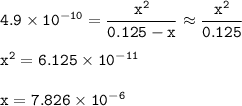Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
How many joules of heat are absorbed to raise the temperature of 650 grams of water from 5.00c to it's boiling point, 100c
Answers: 1

Chemistry, 21.06.2019 22:30
Which of these sequences lists the correct order for the creation of sedimentary rock from sediment? a. deposition, burial, compaction, cementation b. burial, deposition, compaction, cementation c. compaction, deposition, burial, cementation d. cementation, deposition, burial, compaction
Answers: 1

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1
You know the right answer?
15) What is the hydronium ion concentration [H3O + ] of a 0.125 M hydrocyanic acid solution with Ka...
Questions

Mathematics, 21.08.2019 09:00


History, 21.08.2019 09:00



Computers and Technology, 21.08.2019 09:00

Mathematics, 21.08.2019 09:00




Chemistry, 21.08.2019 09:00

English, 21.08.2019 09:00


Health, 21.08.2019 09:00

French, 21.08.2019 09:00





![\large {\boxed {\bold {Ka \: = \: \frac {[H ^ +] [A ^ -]} {[HA]}}}}](/tpl/images/0858/5891/560b5.png)
![\tt Ka=\dfrac{[H_3O^+][CN^-]}{[HCN^-]}](/tpl/images/0858/5891/535c0.png)