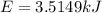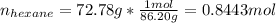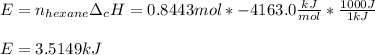The combustion of hexane is given by the following reaction. 2 C 6 H 14 + 19 O 2 → 12 CO 2 + 14 H 2 O The enthalpy of reaction is −4163.0 kJ/mol. How much energy (in joules) will be released if 72.78 grams of hexane is burned. (Molar mass of hexane = 86.20 g/mol).

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 22:30
Joseph has hypothesized that sound travels in waves. if he were following the scientific method, what should he do next? a. ask a question. b. test the hypothesis. c. study the results. d. tell other scientists about his hypothesis.
Answers: 1

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2
You know the right answer?
The combustion of hexane is given by the following reaction. 2 C 6 H 14 + 19 O 2 → 12 CO 2 + 14 H 2...
Questions


History, 02.08.2019 12:20



Geography, 02.08.2019 12:30

Business, 02.08.2019 12:30


English, 02.08.2019 12:30



Biology, 02.08.2019 12:30

Mathematics, 02.08.2019 12:30

Spanish, 02.08.2019 12:30


Social Studies, 02.08.2019 12:30

Chemistry, 02.08.2019 12:30

Mathematics, 02.08.2019 12:30