

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 23.06.2019 08:00
Problem page a jet airplane reaches 846. km/h on a certain flight. what distance does it cover in 13.0 min? set the math up. but don't do any of it. just leave your answer as a math expression. also, be sure your answer includes all the correct unit symbols.
Answers: 2

Chemistry, 23.06.2019 10:00
Why sncl2 is solid while sncl4 is liquid at room temprature explain it in easy way
Answers: 1
You know the right answer?
1s2 2s2 2p6 3s2 3p6 Atoms of an element, X, have the electronic configuration shown above. The compo...
Questions

Mathematics, 04.02.2020 05:55

Biology, 04.02.2020 05:55


History, 04.02.2020 05:55




Biology, 04.02.2020 05:55


Biology, 04.02.2020 05:55



Mathematics, 04.02.2020 05:56

English, 04.02.2020 05:56

Mathematics, 04.02.2020 05:56

History, 04.02.2020 05:56


Biology, 04.02.2020 05:56

Mathematics, 04.02.2020 05:56

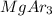 .
.


