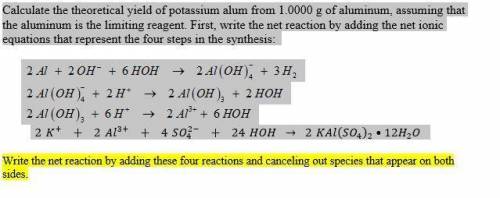
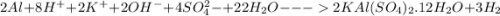Calculate the theoretical yield of potassium alum from 1.0000 g of aluminum, assuming that the aluminum is the limiting reagent. First, write the net reaction by adding the net ionic equations that represent the four steps in the synthesis:
(view image for reactions)
Write the net reaction by adding these four reactions and canceling out species that appear on both sides.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Becquerel expected to find ( he developed the photographic plate that had sun-exposed minerals on top of it. becquerel expected to find ( he developed the photographic plate that had been in the closed drawer.
Answers: 2

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 23:30
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3

Chemistry, 23.06.2019 12:40
Metric temperature is measured in celsius and fahrenheit. true or false
Answers: 2
You know the right answer?
Calculate the theoretical yield of potassium alum from 1.0000 g of aluminum, assuming that the alumi...
Questions


Chemistry, 18.07.2021 01:00

Mathematics, 18.07.2021 01:00


Mathematics, 18.07.2021 01:00




Mathematics, 18.07.2021 01:00

Mathematics, 18.07.2021 01:00


English, 18.07.2021 01:00

Mathematics, 18.07.2021 01:00

Mathematics, 18.07.2021 01:00




Business, 18.07.2021 01:00


Chemistry, 18.07.2021 01:00