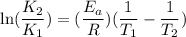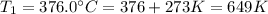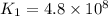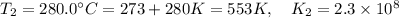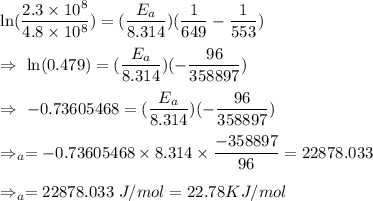The rate constant for a certain reaction is measured at two different temperatures:
Temperature K
376.0°C 4.8 x 10^8
280°C 2.3 x 10^8
Assuming the rate constant obeys the Arrhenius equation, calculate the activation energy for this reaction. Round your answer to significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
What is common about these molecules? a.their atoms are held together by covalent bonds. b.they are all made up of the same two atoms. c.their atoms are held together by ionic bonds. d.they are all made up of oxygen atoms only.
Answers: 3



Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1
You know the right answer?
The rate constant for a certain reaction is measured at two different temperatures:
Temperature K
Questions

Mathematics, 03.04.2020 21:07

Mathematics, 03.04.2020 21:07





History, 03.04.2020 21:08



Mathematics, 03.04.2020 21:08


Engineering, 03.04.2020 21:09

Biology, 03.04.2020 21:09


Biology, 03.04.2020 21:09


Mathematics, 03.04.2020 21:10

Mathematics, 03.04.2020 21:10


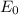 follows by formula:
follows by formula: