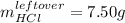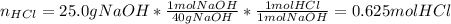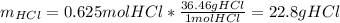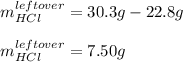Chemistry, 29.10.2020 17:10 AnnaValentine2764
Aqueous hydrochloric acid will react with solid sodium hydroxide to produce aqueous sodium chloride and liquid water . Suppose 30.3 g of hydrochloric acid is mixed with 25. g of sodium hydroxide. Calculate the minimum mass of hydrochloric acid that could be left over by the chemical reaction. Be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The wave shown on the electromagnetic spectrum disturb the medium it passes through a)different frequency. b)the same frequency .
Answers: 2


Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 23.06.2019 05:40
Why is any chemical reaction always balanced? give reasons and explain the easiest way to solve the balancing problems in chemical equations with stoichiometric coefficients upto 20 as hit and trial doesn't always work. give full reasoning
Answers: 1
You know the right answer?
Aqueous hydrochloric acid will react with solid sodium hydroxide to produce aqueous sodium chloride...
Questions

Health, 18.02.2021 18:50



Mathematics, 18.02.2021 18:50

English, 18.02.2021 18:50





Chemistry, 18.02.2021 18:50

History, 18.02.2021 18:50

Mathematics, 18.02.2021 18:50


Mathematics, 18.02.2021 18:50


Social Studies, 18.02.2021 18:50


Social Studies, 18.02.2021 18:50

Biology, 18.02.2021 18:50