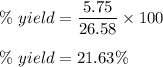Chemistry, 29.10.2020 17:10 alexam2007
When 11.0g of Mg(s) reacts with excess water to produce 5.75g of Mg(OH)2, what is the percent yield of the reaction

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2
You know the right answer?
When 11.0g of Mg(s) reacts with excess water to produce 5.75g of Mg(OH)2, what is the percent yield...
Questions

Mathematics, 17.05.2021 22:20

Mathematics, 17.05.2021 22:20


Mathematics, 17.05.2021 22:20



Chemistry, 17.05.2021 22:20

Mathematics, 17.05.2021 22:20


Mathematics, 17.05.2021 22:20

Geography, 17.05.2021 22:20

History, 17.05.2021 22:20



Mathematics, 17.05.2021 22:20

Mathematics, 17.05.2021 22:20

Mathematics, 17.05.2021 22:20

Arts, 17.05.2021 22:20

Social Studies, 17.05.2021 22:20

Mathematics, 17.05.2021 22:20

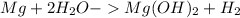
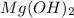
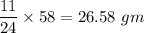 .
.