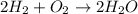Chemistry, 31.01.2020 07:50 sbhunsaker9025
Which of the following is true about the mass of the reactants in a chemical reaction? a. the total mass of the reactants in a chemical reaction will never be equal to the total mass of the products. b. the total mass of the reactants in a chemical reaction will be significantly more than the total mass of the products. c. the total mass of the reactants in a chemical reaction is conserved and will be equal to the total original mass of the products. d. the total mass of the reactants in a chemical reaction will be significantly less than the total mass of the products.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 23.06.2019 06:30
What type of chemical reaction occurs between silver nitrate (agno3) and copper (cu)? the equation i was given is 2agno3 + cu —> 2ag+ cu(no3)2.
Answers: 1

Chemistry, 23.06.2019 07:40
What is the reduction potential of a hydrogen electrode that is still at standard pressure, but has ph = 5.65 , relative to the she?
Answers: 1

Chemistry, 23.06.2019 09:30
The mass of a proton is approximately equal to the mass of
Answers: 1
You know the right answer?
Which of the following is true about the mass of the reactants in a chemical reaction? a. the total...
Questions

Engineering, 24.12.2019 20:31





Computers and Technology, 24.12.2019 20:31