The coinage metals, Cu, Ag, and Au, all lie
in one column of the periodic table. Consider
Ag....

Chemistry, 28.10.2020 14:00 trevorhenyan51
The coinage metals, Cu, Ag, and Au, all lie
in one column of the periodic table. Consider
Ag. An atom of Ag will have a certain ground
state electron configuration according to the
Aufbau principle. The outermost electrons in
Ag will have the configuration
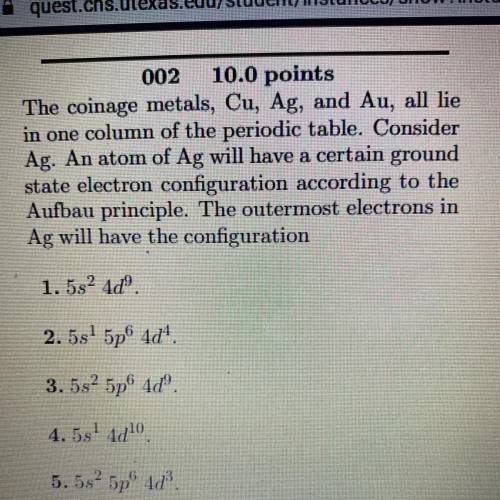

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
In any energy conversion, some of the energy is lost to the environment as question 5 options: electrical energy potential energy sound energy thermal energy
Answers: 1

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3
You know the right answer?
Questions


English, 16.12.2020 19:10

Chemistry, 16.12.2020 19:10

Chemistry, 16.12.2020 19:10




Mathematics, 16.12.2020 19:10




Mathematics, 16.12.2020 19:10


Mathematics, 16.12.2020 19:10



History, 16.12.2020 19:10

Mathematics, 16.12.2020 19:10





