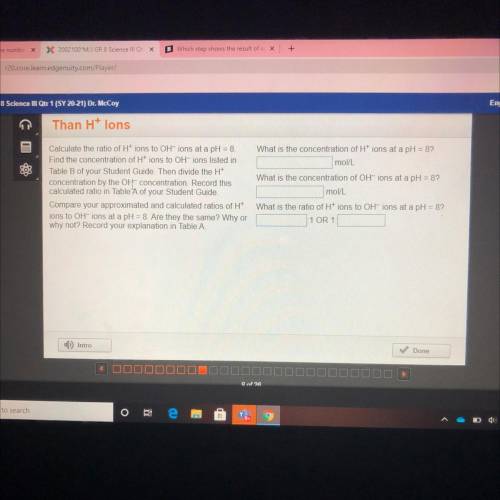
What is the concentration of H+ ions at a pH = 8?
mol/L
Calculate the ratio of Htions to OH-i...

Chemistry, 28.10.2020 06:00 clairebear65
What is the concentration of H+ ions at a pH = 8?
mol/L
Calculate the ratio of Htions to OH-ions at a pH = 8
Find the concentration of H+ ions to OH-ions listed in
Table B of your Student Guide. Then divide the H+
concentration by the OH concentration. Record this
calculated ratio in Table A of your Student Guide.
Compare your approximated and calculated ratios of H+
ions to OH-ions at a pH = 8. Are they the same? Why or
why not? Record your explanation in Table A.
What is the concentration of OH-ions at a pH = 8?
mol/L
What is the ratio of H+ ions to OH-ions at a pH = 8?
1 OR 1.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2
You know the right answer?
Questions





Mathematics, 14.10.2019 16:10

Arts, 14.10.2019 16:10


Biology, 14.10.2019 16:10

Arts, 14.10.2019 16:10


Chemistry, 14.10.2019 16:10

History, 14.10.2019 16:10




Mathematics, 14.10.2019 16:10






