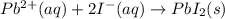Chemistry, 17.09.2019 04:30 kyliegriffis
Considering the following precipitation reaction: pb(no3)2(aq) + 2ki(aq) → pbi2(s) + 2kno3(aq),
which ion(s) would not be spectator ions?
a) pb2+ , no3-
b) k+ , i
c) no3- , pb2+
d) pb2+, i –
e) all the above ions are in the net ionic equation

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the mass of fuel required for the expected energy consumption in the united states for the next ten years. energy use per person per year in the united states = 3.5 x 1011joules base calculations on current population of 310,000,000.
Answers: 2

Chemistry, 21.06.2019 23:00
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1
You know the right answer?
Considering the following precipitation reaction: pb(no3)2(aq) + 2ki(aq) → pbi2(s) + 2kno3(aq),
Questions

Chemistry, 21.01.2021 19:40

History, 21.01.2021 19:40

Mathematics, 21.01.2021 19:40

Mathematics, 21.01.2021 19:40

Biology, 21.01.2021 19:40



Chemistry, 21.01.2021 19:40

Mathematics, 21.01.2021 19:40



Mathematics, 21.01.2021 19:40




English, 21.01.2021 19:40

Mathematics, 21.01.2021 19:40


Mathematics, 21.01.2021 19:40

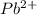 and
and 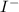
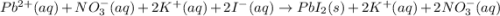
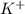 and
and 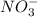 and are spectator ions.
and are spectator ions.