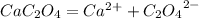Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 10:30
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1

Chemistry, 22.06.2019 23:00
What extra step distinguishes fermentation from glycolysis
Answers: 1

Chemistry, 22.06.2019 23:00
What is formed when amino acids form long chains or polymerize
Answers: 1
You know the right answer?
Calcium oxalate (cac2o4) has a ksp value of 2.3 × 10–9 at 25°c. calculate the molar solubility of ca...
Questions





Geography, 23.06.2019 15:30

Mathematics, 23.06.2019 15:30


English, 23.06.2019 15:30


History, 23.06.2019 15:30

Physics, 23.06.2019 15:30






History, 23.06.2019 15:30


Chemistry, 23.06.2019 15:30