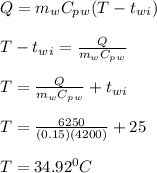Chemistry, 27.10.2020 19:10 Baby010391
A 10.0 g piece of hot metal at 300. °C was dropped into a 150.0 g sample of cooler room temperature water that was initially 25.0 °C. If 6.25 kJ of heat was transferred, what was the final temperature of the water?What was the specific heat of the metal?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1


You know the right answer?
A 10.0 g piece of hot metal at 300. °C was dropped into a 150.0 g sample of cooler room temperature...
Questions

Mathematics, 14.12.2020 21:10

Mathematics, 14.12.2020 21:10




History, 14.12.2020 21:10


Spanish, 14.12.2020 21:10

Mathematics, 14.12.2020 21:10

Mathematics, 14.12.2020 21:10


Mathematics, 14.12.2020 21:10

English, 14.12.2020 21:10

History, 14.12.2020 21:10

Mathematics, 14.12.2020 21:10

Computers and Technology, 14.12.2020 21:10

Mathematics, 14.12.2020 21:10



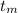 = 300 °C
= 300 °C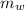 = 150 g = 0.15 kg
= 150 g = 0.15 kg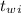 = 25.0 °C
= 25.0 °C