
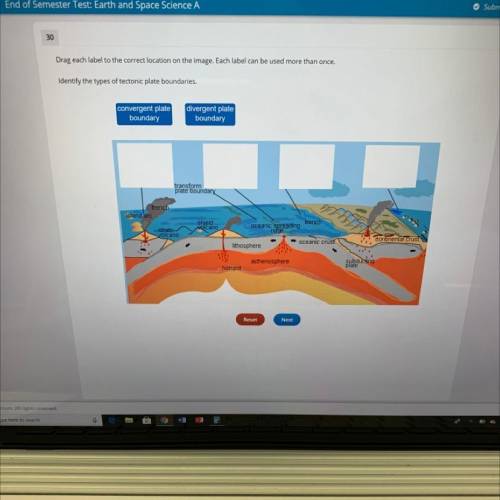
Drag each label to the correct location on the image. Each label can
Identify the types of tectonic plate boundaries.
convergent plate
boundary
divergent plate
boundary
transform
plate boundary
trench
island arc
shjeld
volcano
trench
strato
volcano
oceanic spreading
ridge
oceanic crust
continental crust
lithosphere
asthenosphere
hotspot
subducting
plate

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Which substance absorbs 58.16 kj of energy when 3.11 mol vaporizes? a)ch4 b)h2s c)co2 d)nacl
Answers: 2

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3

Chemistry, 22.06.2019 05:50
Astudent made a graph plotting the progress of a reaction over time. the student forgot to label the y-axis of the graph. a graph is shown with two graph lines. one graph line starts at a higher position on the y axis and slopes downwards towards the right. the other graph line starts at a lower position on the y axis and slopes upwards towards the right. the two graph lines stop short of intersecting each other and continue as separate lines which gradually become straight and parallel to the x axis. a vertical line is shown at a point where the two graph lines finally became parallel to the x axis. this vertical line is labeled equilibrium. the title on the x axis is time and an arrow pointing towards the right is shown above time. the title on the y axis is left blank. what best explains the label that the student should use on the y-axis? amount, because as the amount of product decreases, the amount of reactant increases over time. reaction rate, because forward and backward reaction become equal at equilibrium. amount, because the amounts of reactants and products become constant after equilibrium is reached. reaction rate, as the rate of forward reaction increases and rate of backward reaction decreases over time.
Answers: 3

You know the right answer?
Drag each label to the correct location on the image. Each label can
Identify the types of tectonic...
Questions

Arts, 10.11.2020 23:50

Mathematics, 10.11.2020 23:50

Mathematics, 10.11.2020 23:50

Biology, 10.11.2020 23:50

Biology, 10.11.2020 23:50

Computers and Technology, 10.11.2020 23:50

English, 10.11.2020 23:50

Mathematics, 10.11.2020 23:50

Social Studies, 10.11.2020 23:50

Health, 10.11.2020 23:50



English, 10.11.2020 23:50

Mathematics, 10.11.2020 23:50



Chemistry, 10.11.2020 23:50







