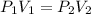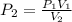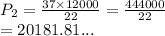Chemistry, 27.10.2020 18:00 cassiuspricerules
A gas sample in a Boyle’s law apparatus is compressed to a volume of 22.0 mL. If its original volume was 37.0 mL at 12,000 Pa, what is the new pressure of the gas sample. Show your calculations.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Which object forms when a supergiant runs out of fuel? a red giant a black hole a white dwarf a neutron star
Answers: 1

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3

You know the right answer?
A gas sample in a Boyle’s law apparatus is compressed to a volume of 22.0 mL. If its original volume...
Questions

Mathematics, 28.10.2020 17:00






Mathematics, 28.10.2020 17:00

Health, 28.10.2020 17:00

Mathematics, 28.10.2020 17:00






Computers and Technology, 28.10.2020 17:00





English, 28.10.2020 17:00