
Chemistry, 27.10.2020 17:20 mvongphakdy8124
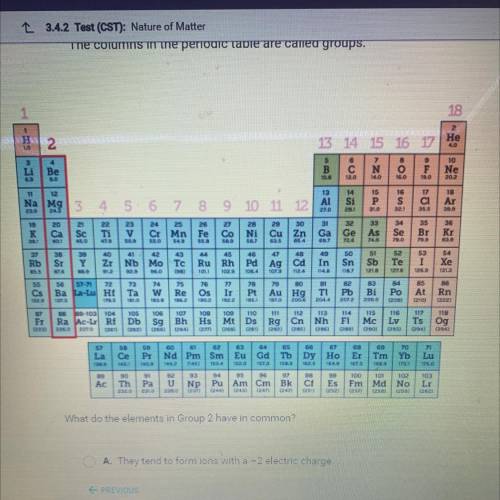
What do the elements in Group 2 have in common?
A. They tend to form ions with a -2 electric charge.
B. They tend to form ions with a +2 electric charge.
C. They are all highly reactive nonmetals.
D. They are all found in nature in their pure forms.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
When curium-242 is bombarded with an alpha particle, two products are formed, one of which is a nudge on. what is the other product
Answers: 3

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1

Chemistry, 22.06.2019 22:30
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1
You know the right answer?
What do the elements in Group 2 have in common?
A. They tend to form ions with a -2 electric charge...
Questions

English, 18.08.2021 18:30

Mathematics, 18.08.2021 18:30







Business, 18.08.2021 18:30





Mathematics, 18.08.2021 18:30

Mathematics, 18.08.2021 18:40

Biology, 18.08.2021 18:40


Mathematics, 18.08.2021 18:40

History, 18.08.2021 18:40



