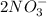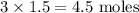When calcium nitrate, Ca(NO3)2, dissolves in water, it dissociates into its ions. We can represent that process by the following equation: Ca(NO3)2(s) → Ca2+(aq) + 2NO3-(aq) When 1.5 mol calcium nitrate dissolves in water, how many moles of ions should be present in solution? Group of answer choices

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 12:30
What type of scientific model does the flow chart represent? (a chart of the scientific process) a. conceptual b. mathematical c. physical d. virtual
Answers: 1

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1
You know the right answer?
When calcium nitrate, Ca(NO3)2, dissolves in water, it dissociates into its ions. We can represent t...
Questions

Social Studies, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30



English, 18.03.2021 01:30




Mathematics, 18.03.2021 01:30


Mathematics, 18.03.2021 01:30



Social Studies, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30




Mathematics, 18.03.2021 01:30

Computers and Technology, 18.03.2021 01:30

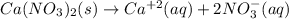
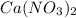 produces 2 moles of
produces 2 moles of 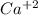 and 1 mole of
and 1 mole of