
Chemistry, 26.10.2020 17:20 justinchou814
Calculate the mass defect for 239U239U, which has a mass of 239.05429 amuamu . (The mass of 11H11H is 1.00783 amuamu, and the mass of a neutron is 1.00866 amuamu .)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1
You know the right answer?
Calculate the mass defect for 239U239U, which has a mass of 239.05429 amuamu . (The mass of 11H11H i...
Questions






English, 13.11.2019 07:31







Computers and Technology, 13.11.2019 07:31







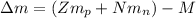
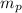 : is the proton mass = 1.00783 amu
: is the proton mass = 1.00783 amu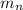 : is the neutron mass = 1.00866 amu
: is the neutron mass = 1.00866 amu ![\Delta m = (Zm_{p} + Nm_{n}) - M = [92*1.00783 amu + (239 - 92)*1.00866 amu] - 239.05429 amu = 1.93909 amu](/tpl/images/0840/4283/ccb79.png)


