Chemistry, 26.10.2020 17:00 ghughes665
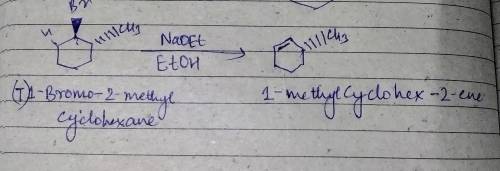
Which product (or products) would be formed in appreciable amount(s) when trans-1-bromo-2-methylcyclohexane undergoes dehydrohalogenation upon treatment with sodium ethoxide in ethanol

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 03:00
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 2

Chemistry, 23.06.2019 05:30
Idont understand it 1.what is the boiling point of a solution of 675 grams of ethylene glycol (c2h6o2) in 2.50 liters of water?
Answers: 2

Chemistry, 23.06.2019 14:00
Tinererining 01: 57: 44 which statement correcte describes the actual veld and the theoretical yield of a reaction? textual vec is calculated to the reactant amounts but the theoretical yeld must be measured for each instance of a the actual vec is calculated from the amount of the limiting reactant and the theoretical yield is calculated from the 发公主 the actual weld depends on the reaction centers, but the theoretical yield and only with reactant amounts the actual vele represents the maximum weld possible and the theoretical yield assumes perfect reaction conditions save and ext e அட
Answers: 2
You know the right answer?
Which product (or products) would be formed in appreciable amount(s) when trans-1-bromo-2-methylcycl...
Questions

Computers and Technology, 13.12.2020 09:10



Mathematics, 13.12.2020 09:10





Mathematics, 13.12.2020 09:10


Arts, 13.12.2020 09:10

Advanced Placement (AP), 13.12.2020 09:10


History, 13.12.2020 09:10

Chemistry, 13.12.2020 09:10




Mathematics, 13.12.2020 09:10