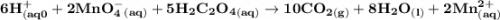Chemistry, 26.10.2020 16:40 Hannahdavy5434
A student dissolved a 0.139g sample of oxalic acid, H2C2O4, in water in an Erlenmeyer flask. Then the student titrated the H2C2O4 solution in the flask with a solution of KMnO4, which has a dark purple color. The balanced chemical equation for the reaction that occurred during the titration is shown above. (a) Identify the species that was reduced in the titration reaction. Justify your answer in terms of oxidation numbers.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 12:40
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2
You know the right answer?
A student dissolved a 0.139g sample of oxalic acid, H2C2O4, in water in an Erlenmeyer flask. Then th...
Questions





Mathematics, 07.02.2022 16:40


Arts, 07.02.2022 16:40

Mathematics, 07.02.2022 16:40


Mathematics, 07.02.2022 16:40



Mathematics, 07.02.2022 16:40



Law, 07.02.2022 16:50



Geography, 07.02.2022 16:50