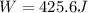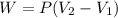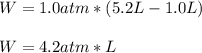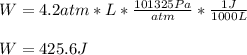Chemistry, 26.10.2020 16:40 lucygperez4099
Calculate the work for the expansion of CO2 from 1.0 to 5.2 liters against a pressure of 1.0 atm at constant temperature.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2

You know the right answer?
Calculate the work for the expansion of CO2 from 1.0 to 5.2 liters against a pressure of 1.0 atm at...
Questions

Mathematics, 14.01.2021 19:20

Mathematics, 14.01.2021 19:20


English, 14.01.2021 19:20

Mathematics, 14.01.2021 19:20




Biology, 14.01.2021 19:20

Physics, 14.01.2021 19:20

Arts, 14.01.2021 19:20

Mathematics, 14.01.2021 19:20




Mathematics, 14.01.2021 19:20


Social Studies, 14.01.2021 19:20

Physics, 14.01.2021 19:20