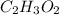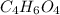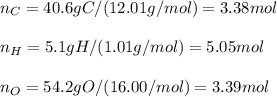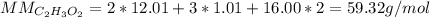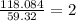Chemistry, 22.10.2020 22:01 tiffanyjadeb
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen.
In an experiment, the molar mass of the compound was determined to be 118.084 g/mol. What is the molecular formula of the compound?
For both questions, show your work or explain how you determined the formulas by giving specific values used in calculations. (10 points)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3
You know the right answer?
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen...
Questions

Social Studies, 22.09.2019 05:00


Business, 22.09.2019 05:00

Chemistry, 22.09.2019 05:00


Mathematics, 22.09.2019 05:00



Mathematics, 22.09.2019 05:00


Mathematics, 22.09.2019 05:00


Mathematics, 22.09.2019 05:00

History, 22.09.2019 05:00


Mathematics, 22.09.2019 05:00


Mathematics, 22.09.2019 05:00