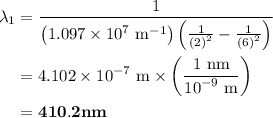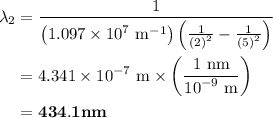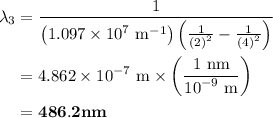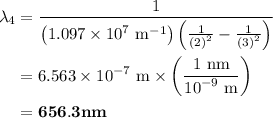Chemistry, 17.09.2019 12:20 lizzyhearts
The spectral lines observed for hydrogen arise from transitions from excited states back to the n=2 principle quantum level. calculate the wavelengths associated with the spectral transitions of the hydrogen atom from the n=6,5,4 and 3 to the n=2 level.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3

Chemistry, 22.06.2019 22:00
8) warming your hands by a fire is an example if which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 1

Chemistry, 23.06.2019 12:20
Describe the structure of ammonium lauryl sulfate. refer to the given diagram. your answer should include the type of bonding, the elements contained, and the size and shape of the molecule. write a short paragraph.
Answers: 3
You know the right answer?
The spectral lines observed for hydrogen arise from transitions from excited states back to the n=2...
Questions

Mathematics, 22.04.2021 14:00

Mathematics, 22.04.2021 14:00

Mathematics, 22.04.2021 14:00

Geography, 22.04.2021 14:00

Mathematics, 22.04.2021 14:00


Computers and Technology, 22.04.2021 14:00

Biology, 22.04.2021 14:00

Mathematics, 22.04.2021 14:00

English, 22.04.2021 14:00

Mathematics, 22.04.2021 14:00


Biology, 22.04.2021 14:00


History, 22.04.2021 14:00

Mathematics, 22.04.2021 14:00


Geography, 22.04.2021 14:00

Mathematics, 22.04.2021 14:00

History, 22.04.2021 14:00

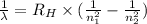
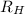 = Rydberg constant =
= Rydberg constant = 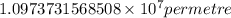
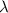 = wavelength
= wavelength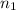 and
and 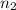 are the level of transitions.
are the level of transitions.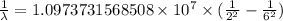
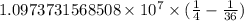
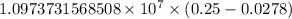
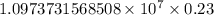
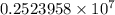
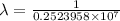


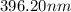
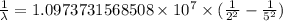
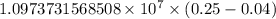
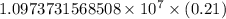
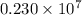
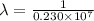


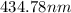
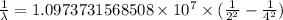
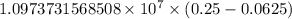
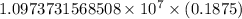
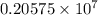
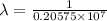


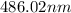
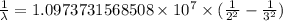
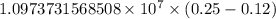
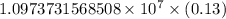
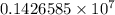
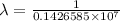


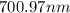
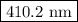 .
.
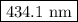 .
.
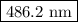 .
.
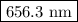 .
.
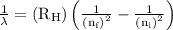 …… (1)
…… (1)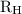 is the Rydberg constant that has the value
is the Rydberg constant that has the value 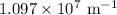 ,
, 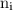 is the initial energy level of transition, and
is the initial energy level of transition, and 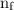 is the final energy level of transition.
is the final energy level of transition.
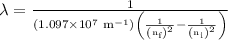 …… (2)
…… (2)