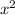Chemistry, 21.10.2020 21:01 absmentalbreakdown
For each compound, write the cation and anion with the appropriate charge. Then write the chemical formula for each compound.
Example: sodium fluoride: Na+1, F-1 NaF
a. Magnesium oxide
b. Strontium iodide
c. Aluminum chloride
d. Calcium phosphide

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1

Chemistry, 23.06.2019 01:00
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3
You know the right answer?
For each compound, write the cation and anion with the appropriate charge. Then write the chemical f...
Questions



Mathematics, 23.11.2020 14:10

Chemistry, 23.11.2020 14:10

Mathematics, 23.11.2020 14:10

Mathematics, 23.11.2020 14:10



Biology, 23.11.2020 14:10

Spanish, 23.11.2020 14:10

History, 23.11.2020 14:10

Biology, 23.11.2020 14:10

Mathematics, 23.11.2020 14:10

Physics, 23.11.2020 14:10


Arts, 23.11.2020 14:10



Health, 23.11.2020 14:10

English, 23.11.2020 14:10