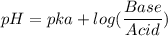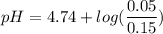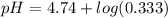Chemistry, 21.10.2020 16:01 anonymous115296
Calculate the expected pH of the buffer after the addition of 1.0 mL of 1M HCl. Remember that you are using 50 mL of the buffer, so be sure to calculate the moles of acetic acid and acetate in 50 mL of the buffer.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1

Chemistry, 23.06.2019 02:00
Which would freeze at a higher temperature: the great salt lake or lake tahoe? a. lake tahoe would freeze at a higher temperature. b. the great salt lake would freeze at a higher temperature. c. both lakes would freeze at the same temperature.
Answers: 2
You know the right answer?
Calculate the expected pH of the buffer after the addition of 1.0 mL of 1M HCl. Remember that you ar...
Questions


History, 01.04.2020 21:59


Chemistry, 01.04.2020 21:59


History, 01.04.2020 21:59

Mathematics, 01.04.2020 21:59



Computers and Technology, 01.04.2020 21:59


Mathematics, 01.04.2020 21:59


Mathematics, 01.04.2020 21:59

Health, 01.04.2020 21:59


Chemistry, 01.04.2020 21:59

Mathematics, 01.04.2020 21:59