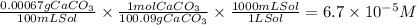Chemistry, 21.10.2020 17:01 landon6663
The solubility of limestone, CaCO3, at 25˚C is 0.00067 g/100 mL. Write the chemical equation for the solubility equilibrium of this sparingly soluble salt in water. Then compute the molar solubility and the solubility product constant Ksp for CaCO3 at 25˚C.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 10:30
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3

You know the right answer?
The solubility of limestone, CaCO3, at 25˚C is 0.00067 g/100 mL. Write the chemical equation for the...
Questions

Mathematics, 17.02.2021 15:30

Biology, 17.02.2021 15:40


History, 17.02.2021 15:40


Mathematics, 17.02.2021 15:40

Computers and Technology, 17.02.2021 15:40

Chemistry, 17.02.2021 15:40

Mathematics, 17.02.2021 15:40

Mathematics, 17.02.2021 15:40

Spanish, 17.02.2021 15:40




Mathematics, 17.02.2021 15:40

Mathematics, 17.02.2021 15:40

Social Studies, 17.02.2021 15:40


Mathematics, 17.02.2021 15:40