
Chemistry, 21.10.2020 16:01 ilovecatsomuchlolol
An aqueous solution of Pb(NO3)2 is made by placing 275 g of solid Pb(NO3)2 into a volumetric flask and adding water to the 1.00 L mark (assume that 775g of water has been added to achieve this total solution volume). (Assume MW of Pb(NO3)2 = 331g/mole) A) What is the molarity (M) of this solution? B) What is the molality (m) of this solution? C) What is the mass % of Pb(NO3)2 in this solution? D) What is the mole fraction of Pb(NO3)2 present in this solution?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1

Chemistry, 22.06.2019 21:50
Answer the questions about this reaction: nai(aq) + cl2(g) → nacl(aq) + i2(g) write the oxidation and reduction half-reactions: oxidation half-reaction: reduction half-reaction: based on the table of relative strengths of oxidizing and reducing agents (b-18), would these reactants form these products? write the balanced equation: answer options: a. 0/na -> +1/na+1e- b. nai(aq) + cl2(g) → nacl(aq) + i2(g) c. +1/na+1e- -> 0 /na d. -1/2i -> 0/i2+2e- e. no f. 4nai(aq) + cl2(g) → 4nacl(aq) + i2(g) g. 2nai(aq) + cl2(g) → 2nacl(aq) + i2(g) h. 4nai(aq) + 2cl2(g) → 4nacl(aq) + 2i2(g) i. nai(aq) + cl2(g) → nacl(aq) + i2(g) j. 0/cl2+2e -> -1/2cl- k. yes
Answers: 1
You know the right answer?
An aqueous solution of Pb(NO3)2 is made by placing 275 g of solid Pb(NO3)2 into a volumetric flask a...
Questions


Biology, 18.11.2020 05:50



Mathematics, 18.11.2020 05:50



Geography, 18.11.2020 05:50

Mathematics, 18.11.2020 05:50

Biology, 18.11.2020 05:50


Mathematics, 18.11.2020 05:50


Arts, 18.11.2020 05:50

Mathematics, 18.11.2020 05:50

History, 18.11.2020 05:50


Mathematics, 18.11.2020 05:50


Biology, 18.11.2020 05:50

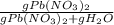 *100%
*100% 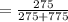 * 100% = 26.2%
* 100% = 26.2%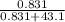 = 0.0189
= 0.0189

