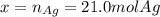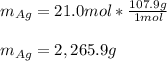Chemistry, 21.10.2020 16:01 thedaisylopez3628
Assume that silver and gold form ideal, random mixtures. Calculate the mass of pure Ag needed to cause an entropy increase of 20 J/K when mixed with 100g of pure Au

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 00:00
The pressure in a fluid is affected by which characteristics of that fluid
Answers: 1

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3
You know the right answer?
Assume that silver and gold form ideal, random mixtures. Calculate the mass of pure Ag needed to cau...
Questions





Computers and Technology, 13.09.2019 01:10



Biology, 13.09.2019 01:10


Mathematics, 13.09.2019 01:10

Computers and Technology, 13.09.2019 01:10


English, 13.09.2019 01:10






Chemistry, 13.09.2019 01:10

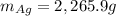
![\Delta S=-n_TR\Sigma[x_i*ln(x_i)]](/tpl/images/0827/7699/ef17c.png)
![\Delta S=-(n_{Au}+n_{Ag})R\Sigma[\frac{n_{Au}}{n_{Au}+n_{Ag}} *ln(\frac{n_{Au}}{n_{Au}+n_{Ag}} )+\frac{n_{Ag}}{n_{Au}+n_{Ag}} *ln(\frac{n_{Ag}}{n_{Au}+n_{Ag}} )]](/tpl/images/0827/7699/1af49.png)
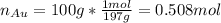
 representing the moles of silver:
representing the moles of silver:![20\frac{J}{mol}=-(0.508+x)8.314\frac{J}{mol*K} \Sigma[\frac{0.508}{0.508+x} *ln(\frac{0.508}{0.508+x} )+\frac{x}{0.508+x} *ln(\frac{x}{0.508+x} )]](/tpl/images/0827/7699/365c1.png)