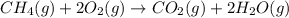Chemistry, 24.08.2019 23:10 hartmaaj95
What element is undergoing oxidation in the following reaction? . ch4(g) +2o2 (g) -> co2(g) +2h2o(g). . option . a) c. b) o . c)h. .

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

You know the right answer?
What element is undergoing oxidation in the following reaction? . ch4(g) +2o2 (g) -> co2(g) +2h...
Questions

Mathematics, 20.06.2020 21:57

English, 20.06.2020 21:57

Mathematics, 20.06.2020 21:57

Social Studies, 20.06.2020 21:57

History, 20.06.2020 21:57

Mathematics, 20.06.2020 21:57



Mathematics, 20.06.2020 21:57

Mathematics, 20.06.2020 21:57

Mathematics, 20.06.2020 21:57




Mathematics, 20.06.2020 21:57

History, 20.06.2020 21:57




Mathematics, 20.06.2020 21:57