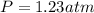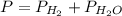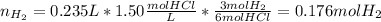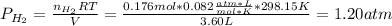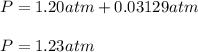Chemistry, 21.10.2020 01:01 hadilalhjajih
2. (6 pts) In a reaction, 235 mL of 1.50 M HCl solution reacts completely with an excess amount of
aluminum. If the hydrogen gas is collected over water in a container with a volume of 3.60 L and at a
temperature of 25.0 °C, calculate the pressure in the container. The vapor pressure of water is 23.78
mmHg (Table 6.4, page 232).
2Al(s) + 6HCl(aq) + 3H2(g) + 2AlCl3(aq)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Based on the law of conservation of energy, which statement is false? answer- energy is lost when machines dont work right
Answers: 1

Chemistry, 21.06.2019 22:30
How many moles are in 250 grams of tungsten (w)? * 4.4x10^23 moles 4.2x10^23 moles 0.7 moles 1.4 moles
Answers: 3

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

You know the right answer?
2. (6 pts) In a reaction, 235 mL of 1.50 M HCl solution reacts completely with an excess amount of...
Questions

Computers and Technology, 27.01.2021 09:00



Mathematics, 27.01.2021 09:00



Mathematics, 27.01.2021 09:00

Business, 27.01.2021 09:00


Mathematics, 27.01.2021 09:00

Social Studies, 27.01.2021 09:00

Mathematics, 27.01.2021 09:00

Mathematics, 27.01.2021 09:00


Spanish, 27.01.2021 09:00



Mathematics, 27.01.2021 09:00

Mathematics, 27.01.2021 09:00

Mathematics, 27.01.2021 09:00