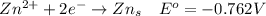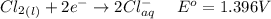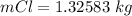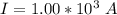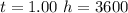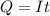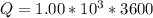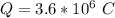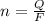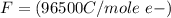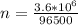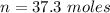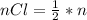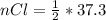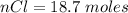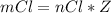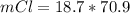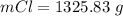Consider the rechargeable battery: Zn(s)0ZnCl (aq)7Cl2(aq)0Cl (l)0C(s) (a) Write reduction half-reactions for each electrode. From which electrode will electrons flow from the battery into a circuit if the electrode potentials are not too different from E 8 values

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Initially, the balloon had 3.0 liters of gas at a pressure of 400 kpa and was at a temperature of 294 k. if the balloon is cooled to 277 k and its volume decreased to 1 l, what will the new pressure in the balloon be?
Answers: 1

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3
You know the right answer?
Consider the rechargeable battery: Zn(s)0ZnCl (aq)7Cl2(aq)0Cl (l)0C(s) (a) Write reduction half-reac...
Questions


Social Studies, 15.07.2021 07:20

Mathematics, 15.07.2021 07:20

Mathematics, 15.07.2021 07:20

Mathematics, 15.07.2021 07:20


Geography, 15.07.2021 07:20





Mathematics, 15.07.2021 07:20




English, 15.07.2021 07:20



Mathematics, 15.07.2021 07:20

English, 15.07.2021 07:20

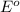 values
values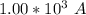 for 1.00 h , how many kg of
for 1.00 h , how many kg of 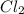 will be consumed
will be consumed