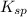Chemistry, 19.10.2020 20:01 dianactorres
Select the term that matches each definition:
a) A decrease in the solubility of an ionic compound as a result of the addition of a common ion.
b) The mass of a salt in grams that will dissolve in 100 mL of water.
c) A solution that has dissolved the maximum amount of a compound at a given temperature. Any further addition of salt will remain undissolved.
d) The product of the molarities of the dissolved ions, raised to a power equal to the ion's coefficient in the balanced chemical equation.
e) The maximum number of moles of a salt that will dissolve in 1 L of solution.
*** Answer options for all questions: ***
- Solubility
- Molar Solubility
- Solubility product constant
- Common ion effect
- Saturated Solution

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
In any energy conversion, some of the energy is lost to the environment as question 5 options: electrical energy potential energy sound energy thermal energy
Answers: 1

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

You know the right answer?
Select the term that matches each definition:
a) A decrease in the solubility of an ionic compound...
Questions

Mathematics, 02.11.2020 20:40

English, 02.11.2020 20:40



Advanced Placement (AP), 02.11.2020 20:40


Arts, 02.11.2020 20:40

Mathematics, 02.11.2020 20:40

Mathematics, 02.11.2020 20:40

English, 02.11.2020 20:40







Arts, 02.11.2020 20:40

Biology, 02.11.2020 20:40

Biology, 02.11.2020 20:40

History, 02.11.2020 20:40