
Chemistry, 19.10.2020 14:01 princessx7543
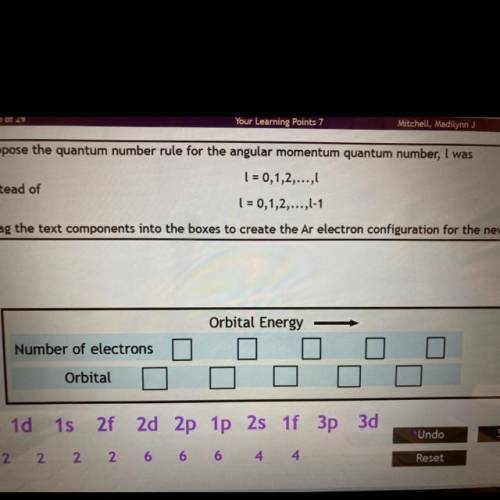
Suppose the quantum number rule for the angular momentum quantum number, I was
l = 0,1,2,...,l
Instead of
l = 0,1,2,...,l-1
Drag the text components into the boxes to create the Ar electron configuration for the new rule.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1
You know the right answer?
Suppose the quantum number rule for the angular momentum quantum number, I was
l = 0,1,2,...,l
Questions

Social Studies, 16.06.2021 07:20

Biology, 16.06.2021 07:20





Biology, 16.06.2021 07:20



Mathematics, 16.06.2021 07:20


History, 16.06.2021 07:20


Spanish, 16.06.2021 07:20

Mathematics, 16.06.2021 07:20


Computers and Technology, 16.06.2021 07:20

Mathematics, 16.06.2021 07:20

History, 16.06.2021 07:20

Spanish, 16.06.2021 07:20



